
The process of titration was started keeping in mind to keep swirling the flask at a constant rate.ġ3. The reaction site, was kept on top of the white tile and 3 drops of methyl orange indicator was added to the 25cm3 solution of substance Z solution.ġ2. 25cm3 of substance Z solution was pipetted out into the Erlenmeyer flask.ġ1. Using the glass funnel, the burette was filled with 0.1M HCl up to the 0.00cm3 mark.ġ0. 0.1M HCl was poured from the stock solution container into the 100cm3 beaker.ĩ. Now, the 50cm3 burette was clamped onto the retort stand.Ĩ. The 250cm3 of substance Z solution was kept aside.ħ. Enough distilled water was added to make a 250cm3 solution in the 250cm3 volumetric flask.Ħ. The solution was poured into the 250cm3 volumetric flask making sure no solid or solution was left behind in the 50cm3 beaker.ĥ. Distilled water was added to completely dissolve the solid mass of substance Z.Ĥ. Using the metal spoon, slowly and carefully, 1.5g of substance Z was weighed out into the 50cm3 beaker.ģ. The 50cm3 beaker was placed on the digital balance and the balance was calibrated to 0.Ģ. To make solutions containing 1.5g, 2.0g and 2.5g of substance Zġ. To titrate substance Z solution against 0.1M HCl. Methyl Orange’s pH of 3.1-4.4 is appropriate for detecting pH of strong acids. To indicate the end-point of the acid-base reaction. To efficiently scoop out small masses of substance Z. To place under the reaction site so that the color change in methyl orange indicator can be easily identified. To hold the burette in place steadily to avoid accidents and inaccuracies. To act as the reaction site and to hold the 25cm3 of substance Z solution. To measure out 25cm3 of substance Z solution into Erlenmeyer flask for titration. To ensure volume of HCl used can be determined precisely since a burette is a precise instrument. To hold 0.1M HCl before pouring into burette. Room temperature – All trials were performed in more or less, the same conditions. Volume of water used to dissolve all samples of substance Z – For all trials, only 250cm3 of distilled water was used.Ĥ. Drops of Phenolphthalein – For all trials, only three drops were used to prevent any misinterpretation of the reaction’s end point.ģ. Molarity of HCl – It was 0.1M for all trials.Ģ. Volume of 0.1M HCl required to neutralize 25.0cm3 of substance Z – The end-point was determined only when the methyl orange indicator completely changed color from yellow to orange.ġ. Volume of substance Z solution – 25.0cm3 of substance Z solution was used for each of the nine trial.ġ. Mass of substance Z – The same digital balance was used to weigh out all the nine samples.Ģ. Substance Z reacts with HCl according to the following balanced chemical equation: Therefore, in order to determine the molecular mass of substance Z, its ability to form alkali solutions was exploited and hence, aqueous samples of substance Z was titrated against 0.1 molarity solutions of HCl.
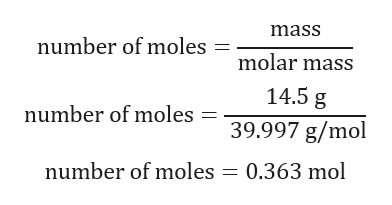
These basic solutions can readily react with strong acids such as HCl to form a salt and water. Alkali metal carbonates are basic in nature and dissolve in water hence forming basic solutions.


 0 kommentar(er)
0 kommentar(er)
